SH-Salmonella Enteriditis Confirmation System
RapidChek® SELECT™Salmonella Enteritidis Confirmation System
Microbiology Pathogenic bacteria LFD Qualitative
Sign-in or register for more informationHave a question or need support?
Do you have questions about our products and/or services? Contact us below.
Contact usDescription & Properties
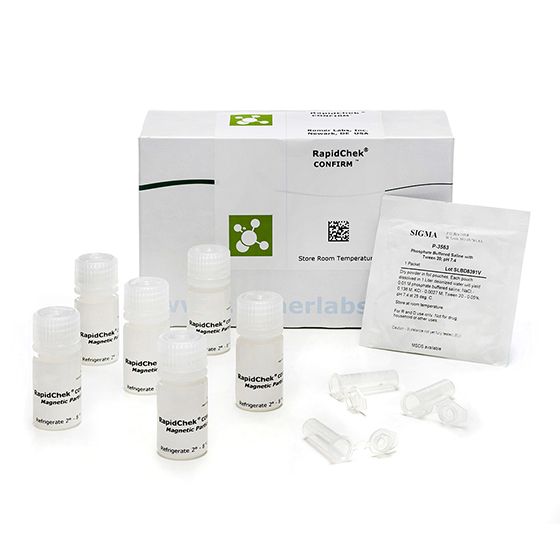
The use of the RapidChek® CONFIRM™ SE IMS kit reduces the presence of other non-SE, Salmonella species through washing steps and concentrates the Group D1 species.
| Microbiology | Pathogenic bacteria |
|---|---|
| Technology/Application | LFD |
| Storage Temperature | 2°C - 8°C |
| Result type | Qualitative |
| Product/Environment | Environmental Monitoring, Product Testing |
Materials
Materials supplied in the standard product
- IMS beads
- PBST packet
- tubes
Materials required but not supplied
- magnetic separation rack
- vortex mixer
Documents
10001400 (7000225):
10001401 (7000226):





