8 Myths of Pathogen Testing

Myth 1
Environmental pathogen testing isn’t improving my food safety program
Most regulations for pathogens require end-product testing before a food production lot can be safely released for distribution and retail. However, there are several challenges associated with the testing of end products.
Firstly, variables such as background microflora, inhibitory characteristics, pH level and salt concentration make pathogen detection difficult. Secondly, sampling methods and sample heterogeneity can lead to inaccuracies, especially if levels of contamination are very low. How can food producers be confident that the number of samples tested is sufficient to ensure that the lot is entirely free of dangerous pathogens? Environmental testing has proven to be a very useful tool in many food safety programs and adapted as part of preventive controls under the FDA Food Safety Modernization Act (FSDA). Environmental monitoring programs (or EMPs) allow a food producer to find growth niches in production areas and take action to eliminate any risk associated with food contamination. Areas with a high risk of pathogen contamination should be sampled, as the purpose of environmental testing is to find positives. The goal of monitoring is to eradicate potential pathogens from the facility and production line before actual contamination takes place, and give food producers an early indication of any contamination risk/problem.
Myth 2
In-house pathogen testing requires substantial investments
Service labs often tell customers that it is too expensive and laborious to test for food pathogens in their own facility. That might be true if standard procedures (FDA BAM, ISO) are used, as these generally require a lot of hands-on time. Other methods such as ELISA and real-time PCR require expensive equipment. In fact, the only equipment you need for in-house tests are an incubator for the enrichment phase and a scale to weigh samples. An autoclave is optional, and only if you wish to sterilize your own media or for waste disposal. There may also be a need for a separate room for enrichment and testing. When using lateral flow strips, there is no need for any extra equipment to interpret the results and therefore, no substantial investment is necessary.
Myth 3
Pathogen testing requires well-trained staff
Rapid methods for pathogens have been streamlined from the days when only highly trained microbiologists could administer such tests, along with countless tubes, plates and a discerning eye. A variety of skill sets are still required, depending on the method but many test kits can be run effectively by trained staff.
Not all test methods require highly educated staff. In many companies, lab staff may have been transferred from the production plant and may not have a scientific degree. Test methods should therefore be simple and robust, with minimal steps to help streamline workflow in the lab and minimize the chance of errors.
A lab must also be able to demonstrate that their staff have been trained in the prevailing test method. Some companies also participate in proficiency test programs to ensure that their lab staff can work independently to obtain accurate test results.
Myth 4
Lab safety requirements deter small companies from conducting their own tests
Food pathogens in the so-called risk group 2, such as Salmonella, Listeria and E.coli O157, can be handled in rooms with some minor adjustments. Pathogens in this risk group are not considered hazardous to laboratory workers and the community in general.
According to the EN 12128 (European Standard), Biosafety in Microbiological and Biomedical Laboratories (US Centers for Disease Control and Prevention) and the World Health Organization (WHO) Biosafety Manual, such rooms should be marked with the biohazard symbol and have access restricted. Only those working with the pathogens should have access to this lab room.
The surfaces in the room need to be resistant to chemicals and therefore also to disinfectants. These lab safety requirements can be easily managed by small food producers.
Myth 5
I cannot dispose of contaminated test material
Yes you can. Here are three options for safe disposal:
1. Use an autoclave and set the temperature at 121°C for at least 20 minutes. The material can now be safely disposed of as residual waste.
2. Use a microwave disinfection system. The material can now be safely disposed of as residual waste.
3. Delegate a waste company for that task. They will order special containers to collect the contaminated waste and discard it for you.
Published on:
Microbiology

Myth 6
There is no need to further analyze samples after a positive test result
All commercially available rapid methods are screening tests and require cultural confirmation after a “presumptive positive” test result. Confirmation requires streaking the enriched test portion to selective agars in order to isolate a typical colony based on morphological and biochemical characteristics. This is especially true when testing end-products or foodstuffs. For environmental samples, cultural confirmation is seldom performed as added sanitation practices are often put in place. There are several options for selective agar plates, with the most common ones found in the USDA Microbiology Laboratory Guidebook (MLG), FDA Bacteriological Analytical Manual (BAM), or ISO reference guides (see Table 1).

Myth 7
There is no reason to test for Listeria species when L. monocytogenes is the adulterant
Listeria monocytogenes (L’mono) may be the only regulated Listeria strain but it is not the only pathogenic one. Listeria ivanovii is another pathogenic strain that may occur in your facility. Another reason for testing Listeria spp. (all Listeria strains), especially in the processing environment, is to track growth niches. This is because L’mono can potentially grow where other Listeria strains thrive. It is a matter of probability whether L’mono is present or not.
If a lot of non-pathogenic Listeria strains (such as Listeria innocua) are present in a sample, they might “overgrow” L’ mono in the enrichment process and potentially cause a false negative result. It is not possible to grow only one Listeria species (i.e., L’mono) during selective enrichment.
Myth 8
All commercially available methods are the same
There are a plethora of rapid methods available on the market today and choosing between them can be daunting. How does a food producer determine which method is best for them? All commercially available rapid methods consist of two parts – an enrichment followed by a detection step. Different media are used during the enrichment phase, using either a conventional or proprietary broth.
The main difference lies in the detection step. Immunoassay methods detect proteins while PCR methods detect DNA. The following evaluation criteria should be considered: inclusivity, exclusivity, sensitivity, specificity, detection limit, reproducibility, repeatability, and certification (see Table 2).
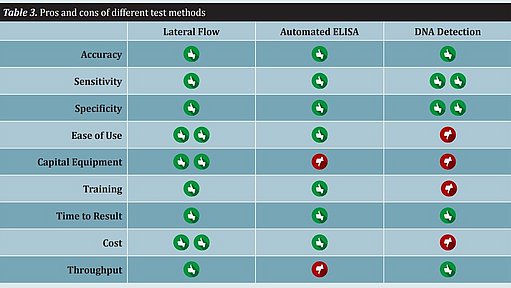
Rapid methods differ significantly with regard to workflow, ease of use, and throughput. Several other features of a test method that should be considered include capital equipment expenditure, training, time to result, and cost. All of these attributes must be taken into consideration when implementing a rapid method. There is no single method that will fit all food producers (see Table 3).