How to Develop an LC-MS/MS-based Multi-Mycotoxin Method

Frequently, cereal crops and raw materials are contaminated with more than one mycotoxin. Romer Labs is developing a single method that can detect multiple mycotoxins simultaneously. Irene Hahn describes how.
There are approximately 400 compounds of low molecular weight that are recognized as mycotoxins, each with different toxic effects for humans and animals. The high number of possible contaminants, as well as repeated reports of mycotoxin co-occurrence makes it necessary to develop suitable detection methods, like liquid chromatography – mass spectrometry (LC-MS/MS)-based multi-mycotoxin methods, to simultaneously analyze several mycotoxins. Developing such methods is challenging because including multiple toxins with different chemical properties in one analysis means that compromises must be made when choosing optimal method parameters.
Published on:
Mycotoxin
Analysis of mycotoxins based on LC-MS/MS
Analytical methods based on reversed-phase LC coupled to MS (LC-MS/MS) have become a powerful and state-of-the-art technique in the qualitative and quantitative analysis of mycotoxins over the last decade.
Advantages of this method are the high sensitivity and selectivity, the application to multi-mycotoxin analysis as well as the delivery of additional information about mass-to-charge ratios (m/z) and fragment ions of the investigated analytes.
Currently, there is a strong trend towards the application of multi mycotoxin methods achieved by LC-MS/MS. The simultaneous determination of a wide range of mycotoxins belonging to different chemical families within one single measurement can be achieved using this technique.
However, issues like the chemical diversity of the compounds themselves, the wide range of agricultural commodities being tested, varying concentration ranges and different occurrence distributions all challenge method development and optimization. Therefore, compromises must be made in the choice of extraction solvent and mobile phase, and conditions may be far from optimal for certain analytes, which include acidic (fumonisins), basic (ergot alkaloids), as well as polar (moniliformin, nivalenol) and apolar compounds (zearalenone, beauvericin). In addition, the lack of suitable commercially available analytical standards for certain analytes results in only qualitative screening statements rather than quantitative results.
Development of a multi-mycotoxin LC-MS/MS method
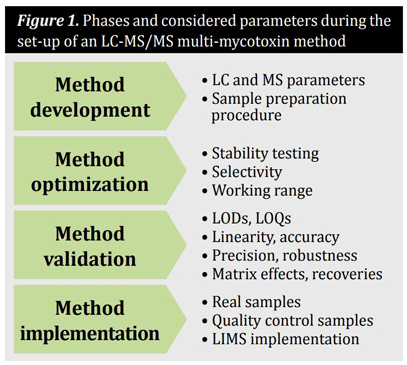
In general, methods for the quantification of mycotoxins in cereals and cereal-based products are comprised of a representative sampling, the optimized sample preparation procedure and a clean-up step as well as the analytical technique including separation and detection.The set-up of a multi-mycotoxin method based on LC-MS/MS follows usually a four-phase process. These and the considered parameters are summarized in Figure 1.
During method development and optimization, the parameters significantly influence the quality and reliability of the results and should be evaluated carefully. For this purpose, analytical standards of each compound with stated purity must be used. However, for certain analytes, analytical standards are not commercially available. In those cases, it might be possible to access standards that are still under research, or work with available material that is less well characterized.

Method development
During the development of an LC-MS/MS based method, the MS and LC parameters, as well as the sample preparation procedure must be worked out. For the optimization of the MS parameters, each compound should be injected as a pure analytical standard directly into the mass spectrometer. Subsequently, the idealionization mode (positive or negative), the most abundant precursor and product ions as well as the ideal declustering potentials, collision energies and collision cell exit potentials must be evaluated. During the optimization of the LC parameters, the ideal mobile phases and gradient as well as the optimal chromatographic column have to be evaluated. The method of choice for sample preparation procedure when analyzing multiple mycotoxins is a dilute-and-shoot approach without any sample cleanup so as not to adulterate the mycotoxin pattern during sample preparation.
For instance, a cleanup (e.g. solid phase extraction) which does not suppress any of the required analytes is rarely available. Nevertheless, if a cleanup is not used, co-eluting and interfering matrix components may affect the ionization efficiency of target analytes resulting in poorer repeatability and lower accuracy. Therefore, the determination and compensation of such matrix effects is essential. This can be achieved by determination of the apparent recovery followed by a correction of the results with this value, matrix-matched calibration or the use of isotope-labeled internal standards. The latter will lead to results with the highest accuracy and reliability, yet with minimal time and cost investment.
Method optimization
The optimization of the analytical method includes stability testing of the analytes in standard solution and samples, as well as proofing selectivity and determination of the working range.
Method validation
Method validation is a prerequisite for the production of reliable results in terms of comparability and traceability. Method validation must be performed separately for each target analyte in all required matrices. Typical performance characteristics that should be evaluated during validation of a quantitative method are limits of detection (LOD), limits of quantification (LOQ), linearity, precision, selectivity, robustness, accuracy, matrix effects and recoveries. The validation of the method can be performed by spiking blank samples with each required analyte at a range of concentrations in replicate. When available, the trueness of the method should be confirmed using certified reference materials. Furthermore, matrix matched materials and the participation in proficiency testing, enable additional quality assurance. Among others, the limited number of reference materials is responsible for the semi-quantitative character of such screening methods. Although multi-toxin methods are already implemented in routine analysis, the high investment and maintenance costs must be considered.
Method implementation
During the implementation, real samples as well as quality control samples should be measured with the validated method. In addition, the validated method should be implemented and used in routine laboratories, which may be difficult in terms of availability of laboratory staff and instrumentation.
General challenges for the determination of mycotoxins
Usually, mycotoxin contaminations are heterogeneously distributed in agricultural crops and may be concentrated in ‘hot-spots’. Therefore, sampling represents an important and crucial step as a representative sample is essential for the precise and accurate determination of mycotoxin levels. With the majority of analytical techniques, a direct detection of mycotoxins in milled cereal samples cannot be achieved and therefore sample preparation procedures are required.
Another important step is the sample extraction. During a conventional solid-liquid extraction, mycotoxins are extracted from ground cereal samples by mechanical shaking with different mixtures of solvents (aqueous and organic), sometimes also with acidic or alkaline modifiers. The resulting extracts can then be further used for analysis. Most of the developed analytical methods for regulatory and scientific purposes are based on chromatographic separation, mainly liquid chromatography (LC), in combination with a variety of detectors. LC detectors for the continuous monitoring and detection of analytes eluting from the chromatographic column are based on measurements of UV/Vis-absorbance, fluorescence and mass spectrometry (MS). Due to the different chemical and physicochemical properties of mycotoxins, the majority of such analytical methods have been optimized for one target compound, or at best, a group of closely related mycotoxins. In addition, these targeted methods often include extraction and cleanup steps to reduce or eliminate unwanted matrix components. Hence, the generated occurrence estimation always depends on the analyzed samples as well as the mycotoxins covered with the used analytical methods.
Conclusion
In conclusion, the development of a multi-mycotoxin method based on LC-MS/MS is challenging when aiming to achieve reliable and comparable quantitative data. A vast number of different parameters that significantly influence the quality and reliability of the results must be considered carefully for each analyte in each matrix separately. In addition, the chemical diversity of the mycotoxins means that compromises have to be made during method development, which may be far from optimal for certain analytes. Moreover, the wide range of agricultural commodities as well as the varying concentration ranges and different occurrence distributions further challenge method development and optimization. Nevertheless, the development of multi-mycotoxin methods is certainly required and advances in this technology will further extend its application.