To Label or Not to Label? Understanding the Proposed Changes to the CODEX Standard for Allergen Labelling

The labelling of food allergens in pre-packaged foods plays a key role in protecting food allergic individuals, as no practical preventative clinical treatment is currently available. The list of major foods and ingredients known to cause hypersensitivity was first included in the Codex General Standard for the Labelling of Pre-packaged Foods (GSLPF) in 1999 (section 4.2.1.4), which mandated their declaration on labels whenever present.
Since the GSLPF was initially drafted, our understanding of food allergens and their management has advanced significantly. In response to a new request from Codex for scientific advice, including current evidence of consumer understanding of allergens, the FAO and the WHO convened a series of three expert meetings from late 2020 to early 2022 to provide scientific advice on three different subjects:
1. Review and validate (or update) the Codex priority allergen list
2. Review and establish threshold levels of the priority allergens in foods
3. Review and establish precautionary labelling (PAL) of the priority allergens in foods
In this article, we consider and discuss the issues that confronted the participants of each meeting and the principal recommendations that resulted. These recommendations reflect more than 20 years of advances both in our understanding of the science of food allergens and in practical responses to the risks posed by food allergens.
Published on:
Food Allergens
Main takeaways from all three
FAO and WHO expert meetings:
Meeting 1: Recommended changes to the global priority allergen list: Addition of sesame and removal of soybean and oats
Meeting 2: Reference Doses (RfD) for priority allergens should be derived from the ED05, i.e., the dose predicted to result in objective reactions in no more than 5% of the population allergic to each of those priority allergens.
Meeting 3: The decision about whether or not to use a PAL statement should form part of a regulatory framework.

Meeting / Report 1: Review and Validation of Codex Alimentarius Priority Allergen List through Risk Assessment
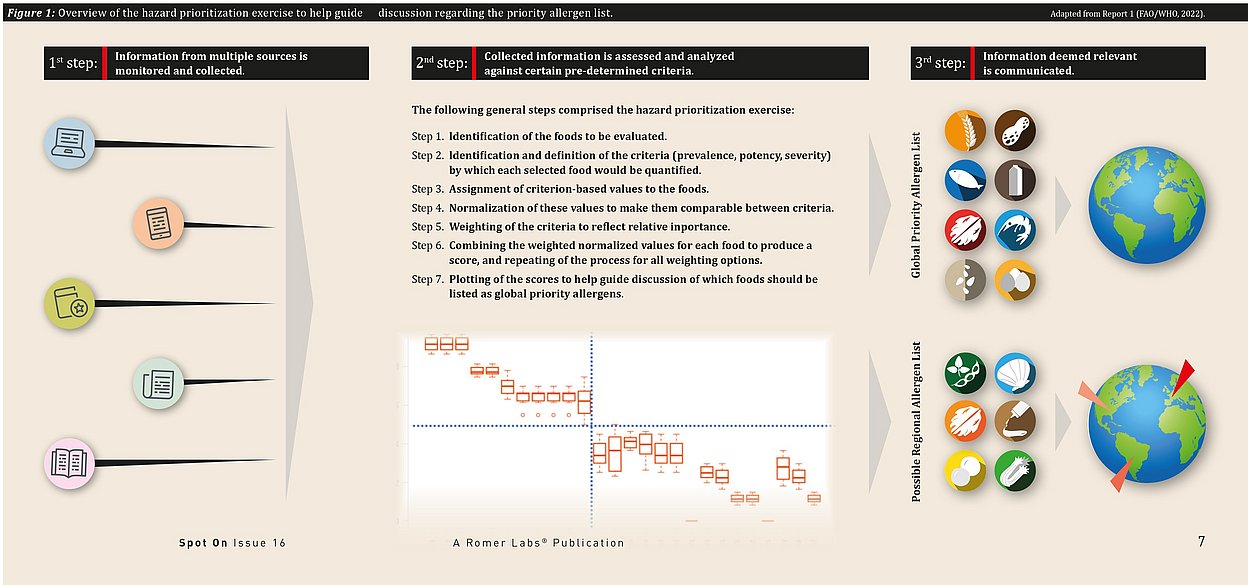
In the first meeting, the Expert Committee stressed that the list should consist only of substances known to provoke well-characterized immune-mediated reactions, i.e., IgE-mediated reactions and coeliac disease. Not only did they further elaborate the criteria for identifying priority allergens, including prevalence of allergy to them, potency, and the severity of observed reactions, they also stressed the importance that those criteria be observed globally.
They undertook a hazard prioritization exercise using a multi-criteria analysis approach, which enabled them to synthesize the different types of data into a single score reflecting the global public health importance of each allergenic food. Additionally, the review process at the core of this prioritization provided transparency and repeatability, not only facilitating scientific review but also providing an approach that could be readily used to rank a putative allergen in terms of public health impact at a global or more local level.
Based on the outcome of the hazard prioritization exercise and the subsequent discussion, the following update to GSLPF section 4.2.1.4 was recommended.
It is important to remember that these recommendations are still being discussed within Codex committees and it can take time before they are accepted or rejected. Additionally, even if they are taken up at the Codex level, it may take some time before changes are seen in local legislation.
Finally, we should emphasize the soundness of the results of the original FAO Technical Consultation on Food Allergies (Rome, 1995) that identified the foods that were eventually included in GSLPF section 4.2.1.4 as adopted in 1999. It is impressive that the list of priority allergens recommended in 1995 is still largely recognizable in the list nearly 30 years later and shows only a few recommended refinements.
Meeting 2: Review and establish threshold levels in foods of the priority allergens
At Meeting 2, the Expert Committee addressed the remit provided by Codex regarding the establishment of thresholds, reviewing the available clinical data and recommending Reference Doses (RfDs) for the foods on the global priority allergen list established in Meeting 1.
The Expert Committee considered both the proportion of people with a relevant allergy who will be affected by exposure to any given amount of allergen (ED01 vs ED05) and the characteristics of any reaction that may occur, summarized as “severity.” The Expert Committee identified and reviewed previously published eliciting dose (ED) values (Remington et al., 2020; Houben et al., 2020) and was also able to review newly available clinical data regarding severity of allergic reactions at low doses (Patel et al., 2021; Turner et al., 2022).
These RfDs could be used either in a risk assessment framework to determine whether to apply precautionary allergy labelling (PAL) or in a risk management decision-making process for allergen-related incidents. Adoption of these RfDs across geographic regions provides a realistic, achievable target that would help to harmonize risk assessment and risk management for food allergens and could potentially make life simpler for stakeholders (consumers, producers, regulators, healthcare practitioners, etc.).
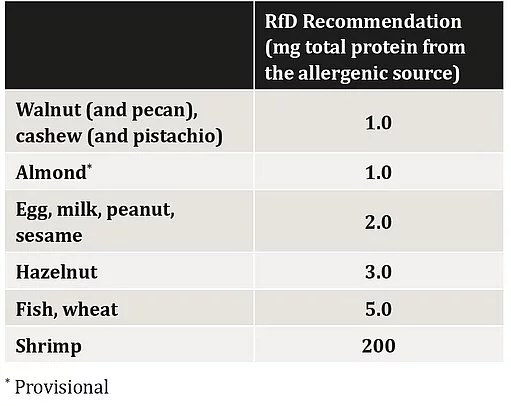
The Expert Committee agreed as a general principle that the RfD values should be contextualized (including potential analytical limitations in verifying them), taking into account the wider and unintended consequences (e.g. PAL appearing on every product due to too stringent recommendations). Importantly, the Expert Committee concluded that a guiding principle should be the consideration of whether selecting a more stringent (lower) ED value would materially improve the impact on public health. This, combined with the completed review of clinical data, severity characterization, and analytical considerations, led the Committee to agree that the safety objective would be met by deriving the RfD from the ED05, or the dose predicted to result in objective reactions in no more than 5% of the allergic population (with rounding) (see RfD recommendation table).
What about food allergen analysis?
The recommended RfDs are a step forward for allergen risk assessment and risk management. However, the analytical community will need to actively support enhancement of method performance and the Expert Committee noted that:
- An RfD [based on the ED05] can be implemented and monitored to some degree with current analytical capabilities but significant limitations in method performance remain.
- They strongly recommended that expression of analytical results be standardized as mg total protein from the allergenic food per kg food product analyzed in order to facilitate result interpretation and comparison with an RfD and action level by users of analytical services.
- To address deficiencies in analytical methodology, they recommended the development of method performance criteria as well as more extensive provision of accessible reference materials for priority allergens.
Meeting 3: Review and establish precautionary labelling (PAL) of priority allergens in foods
Meeting 3 reviewed the current status of PAL and proposed a framework to improve upon its application. The following summarizes their findings:
Current status
- The use of PAL is voluntary and often misunderstood by consumers and the industry.
- The decision to apply PAL (or not) is not part of a standardized risk assessment process.
- Consumers find the information currently provided by PAL to be incomplete, confusing and ambiguous concerning the characteristics of the risk and the action expected of the allergic consumer.
Proposed framework
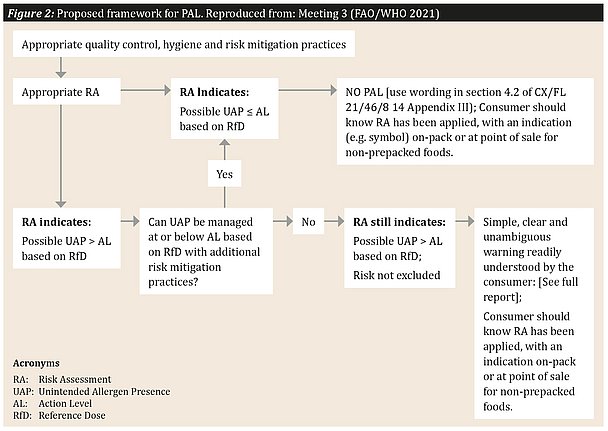
- The Expert Committee recommended that the decision about whether to use a PAL statement should form part of a regulatory framework. This would require food business operators (FBOs) to use PAL only when an unintended allergen presence (UAP) exceeds the relevant RfD.
- Consumers with food allergies should be able to identify on-pack if a qualified risk assessment has been completed, irrespective of whether PAL is used or not.
- A consistent and harmonized approach is the most effective use of PAL for communicating to consumers with food allergy about the risk from UAP.
See Figure 2 for the PAL framework proposed by the Expert Committee. Again, the adoption of such a framework is intended to harmonize the risk analysis for food allergens and make life simpler for all stakeholders.
Concluding remarks
The recent FAO/WHO Expert Consultation represents the largest Codex-driven activity on the topic of food allergies since the inception of the original Codex priority allergen list in 2000. It represents over 120 hours of virtual meetings by up to 25 scientists from across the world with expertise spanning fields as diverse as protein and analytical chemistry, food science, risk assessment and clinical allergology. In addition, countless hours were spent reviewing and preparing syntheses of pertinent data outside those meetings as well as writing the reports. The work built on and integrated the outcomes of considerable amounts of research undertaken over the last two decades, involving many of the participants in the FAO/ WHO consultation in the context of large multinational projects (e.g. Europrevall, iFAAM, the VITAL™ program).
Of course, as with all scientific endeavors, the work does not end, but the outcomes of the Consultation form a major milestone in the development of allergen risk assessment, and thereby in protecting all who suffer from food allergies. All stakeholders will need to be involved in delivering the full value of the work undertaken over the coming years as the recommendations are still being discussed within the Codex Committee on Food Labelling (CCFL) – this can take time and does not mean that the recommendations will be adopted either in full or partially. Even if they are adopted at the Codex level, it may take some time before changes are seen in local legislation.
Until that time comes, all stakeholders need to continue to be vigilant to protect those suffering from food allergies and continue to push for resolutions to the current ambiguities related to allergen risk management and labelling.