Top Three Challenges in External Accreditation Audits

External audits for ISO 17025 accreditation can be a true test of expertise. The auditors typically work in the same field and are themselves genuine experts. They assess the technical competence of the laboratory in-depth. For most labs traceability, measurement uncertainty and matrix effects pose the biggest challenges during an external accreditation audit.
Published on:
Mycotoxin
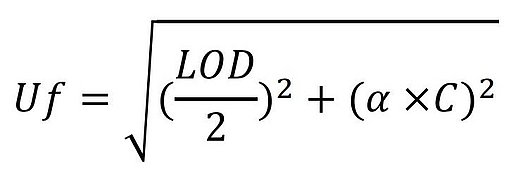
1. Traceability
This is one issue every laboratory struggles with. An accredited laboratory is required to show that a result on one of its test reports can be traced back to international standard (SI) base units. In the case of a result stated in μg/kg (microgram per kilogram) obtained by HPLC (High Performance Liquid Chromatography) or LC-MS (liquid chromatography with mass spectrometry detection) this implies clear documentation. Both use a liquid standard for calibration, and in order to claim full traceability back to the SI base unit kilogram, auditors will want a certification report stating the full procedure of preparation and all measures taken by the supplier. (For more, see the box text on 'Traceability and Certified Reference Materials')
2. Measurement uncertainty
Under ISO 17025 requirements, the measurement uncertainty (mu) of every accredited method must be calculated and included in the test report. There are many ways to estimate or calculate measurement uncertainty and which way is accepted depends a lot on the preference of the national accreditation body. A simple and practical way for small laboratories to estimate measurement uncertainty is through the use of control charts. It is good laboratory practice to use a matrix-based control sample that ideally is naturally contaminated with or has been spiked with the analyte of interest. This sample is then added to each sequence run.
Results of the control samples are plotted on control charts that are used for long-term assessment and identification of trends for each method. The measurement uncertainty is derived from a two-sigma standard deviation of all results. In Europe, a valid approach is the “fitness for purpose” approach published in Commission Regulation (EC) No 401/2006 which details the methods of sampling and analysis for the official control of mycotoxin levels in foodstuffs. The following formula presents a way of calculating the maximum standard uncertainty:
where:
- Uf is the maximum standard uncertainty (μg/kg)
- LOD is the limit of detection of the method (μg/kg)
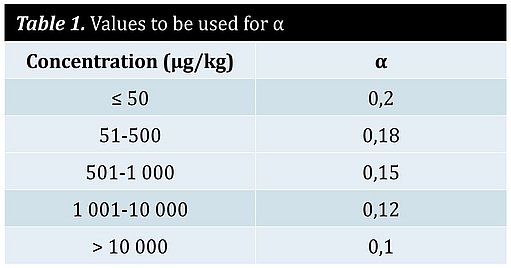
- α is a constant, numeric factor to be used depending on the value of C.
- C is the concentration of interest (μg/kg)
Another approach is to follow the steps described in JCGM 100:2008 Guide to the Expression of Uncertainty in Measurement (GUM) shown in Table 2. Accordingly, uncertainty in a measurement is a result of our incomplete knowledge of the value of the measured quantity in combination with the factors influencing it. Table 3 lists many possible sources of uncertainty in measurement.

3. Matrix effects
Accredited service labs typically face the daily challenge of receiving samples of various matrices. These laboratories validate their methods for the most common matrices. However, even the analysis of an apparently simple matrix like maize is highly influenced by different corn varieties. More complex samples like compound feed or highly processed food can alter analysis results tremendously. Using 13C-labeled internal standards for every analyte and a calculation based on an internal calibration are the most accurate, state-of-the-art approaches in use today. (For more, see the article on 'Overcoming LC-MS/ MS Matrix Effects for Maximum Reliability')

Certification ≠ Accreditation
The terms certification and accreditation are often confused. Whoever wants to certify something, whether it is a management system, a product or a person, needs to be accredited for this task by a national accreditation body. Acquiring ISO 17025 accreditation allows for the issuance of relevant certifications in many jurisdictions.
Conclusion
Traceability, measurement uncertainty and matrix effects pose the biggest challenges for most labs during an external accreditation audit. A certification report from an accredited supplier goes a long way to demonstrate traceability. Several methods are available to calculate measurement uncertainty. However, only the 13C-labeled internal standards can fully correct for matrix effects. With these tools in hand, laboratories will be better positioned to successfully navigate the audit process.